What happens when we change the written form of a chemical reaction that describes an equilibrium? This is usually necessary when we're looking at reaction mechanisms or other stepwise processes.
Multiplying a reaction:
Consider the reaction 2A↔B with K=4. We can multiply that reaction by 3 to get the reaction 6A↔3B. Fundamentally, this is the same reaction and the same process, but what about the equilibrium constant? Writing out the equilibrium constants for both reactions:
So if K = 4, K' = 43 = 64
Changing direction:
If we reverse a chemical reaction, we once again have to adjust the equilibrium constant. Changing 2A↔B to B↔2A means that we are changing the identity of reactants and product. This means that the concentrations that used to be in the numerator of the equilibrium constant are now in the denominator and vice versa. Mathematically, this has the effect of inverting the value of the equilibrium constant. If K = 4 for 2A↔B, then K"=1/4 for B↔2A.
Multipart/Multistep:
For a multistep process, the equilibrium position of each step will affect the overall equilibrium of the system. This is in contrast to kinetics where the rate law of the overall process relies only upon the rate law of the slowest step. For a stepwise process, the equilibrium constant for the overall process is equal to the product of the individual steps.
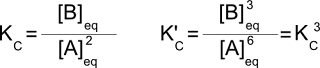
No comments:
Post a Comment