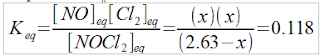Hmm, not sure I need to say more than what's in the title of the post. I submitted grades to the eServices system, they'll be visible to you whenever all the correct electrons flow through all the correct wires to make the interwebs update to display your grades.
I hope everyone had a useful class this summer, let me know if you have any questions and enjoy the rest of your summer.
Info and advice to help General Chemistry students (and anyone interested in chemistry)
2012-07-26
2012-07-24
Corrections to Exam 4 key
Typing up the key late at night was not a good idea... There were a number of errors on page 4 of the Exam 4 key that I posted this morning. If you already looked at the key or printed it out, throw away page 4 and go get a fresh version. Sorry about that. http://www.drbodwin.com/teaching/exams/c210pe4ak.pdf
Exams posted
All the exams we've taken for the summer 2012 class and their keys are posted on my web page, both on the "General Chemistry" page (http://www.drbodwin.com/teaching/genchem.php) and in the "Exam Archive" (http://www.drbodwin.com/teaching/examarchive.php). Let me know if you have questions.
2012-07-23
Redox reactions
We looked at a few redox reactions today in class, some of you wanted the solutions posted. Enjoy.
For each pair of half cells, write the balanced spontaneous (standard) reaction and calculate the spontaneous (standard) cell voltage. {Actually, you'd do the secondpart of that process before you could do the first part}
Cu+1|Cu (E0red= +0.521V) and Fe3+|Fe2+(E0red= +0.771V)
Cu half must be reversed to be the oxidation half rxn
E0cell = E0red+ E0ox = 0.771V + (-0.521V) = +0.250V
Cu(s) ⇄ Cu+1(aq) + 1e⊖(aq)
1e⊖ + Fe3+(aq) ⇄ Fe2+(aq)
------------------------------------------------------------------
Cu(s) + Fe3+(aq) ⇄ Fe2+(aq) + Cu+1(aq)
BrO3-1|Br2(E0red= +1.478V) and Pt2+|Pt (E0red= +1.188V)
Pt half must be reverse to be the oxidation half rxn
E0cell = E0red+ E0ox = 1.478V + (-1.188V) = +0.290V
5 { Pt(s) ⇄ Pt+2(aq) + 2e⊖(aq) }
12 H⊕(aq) + 10e⊖(aq) + 2 BrO3-1(aq) ⇄ Br2(l) + 6 H2O(l)
------------------------------------------------------------------
12 H⊕(aq) + 5 Pt(s) + 2 BrO3-1(aq) ⇄ Br2(l) + 5 Pt+2(aq) + 6 H2O(l)
Cr+2|Cr (E0red= -0.403V) and Pb+4|Pb+2(E0red= +1.69V)
Cr half must be reverse to be the oxidation half rxn
E0cell = E0red+ E0ox = 1.69V + (0.403V) = +2.09V
Cr(s) ⇄ Cr+2(aq) + 2e⊖(aq)
2e⊖ + Pb+4(aq) ⇄ Pb+2(aq)
------------------------------------------------------------------
Cr(s) + Pb+4(aq) ⇄ Pb2+(aq) + Cr+2(aq)
TeO2|Te (E0red= +0.604V) and Se|H2Se (E0red= -0.115V)
Se half must be reverse to be the oxidation half rxn
E0cell = E0red+ E0ox = 0.604V + (0.115V) = +0.719V
2 { H2Se(aq) ⇄ Se(s) + 2e⊖(aq) + 2 H⊕(aq) }
4 H⊕(aq) + 4e⊖ + TeO2(s) ⇄ Te(s) + 2 H2O(l)
------------------------------------------------------------------
2 H2Se(aq) + TeO2(s) ⇄ Te(s) + 2 Se(s) + 2 H2O(l)
MnO4-1|Mn+2(E0red= +1.23V) and ClO4-1|ClO3-1(E0red= +1.201V)
Cl half must be reverse to be the oxidation half rxn
E0cell = E0red+ E0ox = 1.23V + (-1.201V) = +0.03V
5 { H2O(l) + ClO3-1(aq) ⇄ ClO4-1(aq) + 2e⊖(aq) + 2 H⊕(aq) }
2 { 8 H⊕(aq) + 5e⊖ + MnO4-1(aq) ⇄ Mn2+(aq) + 4 H2O(l) }
------------------------------------------------------------------
616H⊕(aq) + 5 H2O(l) + 2 MnO4-1(aq) + 5 ClO3-1(aq) ⇄ 5 ClO4-1(aq) + 2 Mn2+(aq) + 38H2O(l) + 10 H⊕(aq)
6 H⊕(aq) + 5 H2O(l) + 2 MnO4-1(aq) + 5 ClO3-1(aq) ⇄ 5 ClO4-1(aq) + 2 Mn2+(aq) + 3 H2O(l)
2012-07-12
Titrations are AWESOME!!
We looked at titrations today. Remember, titrations are just stoichiometry problems applied to a specific system/type of problem, they're not completely new information, approach them the same way you would approach any other stoichiometry problem:
1. Write a balanced chemical equation
2. Convert whatever you know the most about to moles
3. Using the mole ratio from the balanced chemical equation, convert moles of what you know to moles of what you're looking for
4. Convert moles of what you're looking for into whatever you want to know about it (grams, volume, concentration, etc.)
5. Check that your answer is reasonable (if possible)
On to today's problems...
30.00mL of 0.713M HNO2(aq) is titrated to the equivalence point with 28.43mL of NaOH(aq) of an unknown concentration. What is the concentration of the NaOH(aq) stock solution? What was the pH of the HNO2(aq) solution before the titration begins? What is the pH at the equivalence point? {Ka(HNO2) = 4.0x10-4}
HNO2(aq) + NaOH(aq) ⇄ H2O(l) + NaNO2(aq)
(0.03000L HNO2(aq)) (0.713M HNO2(aq)) = 0.02139mols HNO2
(0.02139mols HNO2) (1mol NaOH / 1mol HNO2) = 0.02139mols NaOH
(0.02139mols NaOH) / (0.02843L NaOH(aq)) = 0.752M NaOH(aq)
NaOH(aq) should be slightly more concentrated than HNO2(aq), so this answer is reasonable
Before the titration begins, this is an aqueous solution of a weak acid, so we can calculate the pH using a Ka-type approach. Setting up a table...
-
HNO2(aq) +H2O(l) ⇄H3O+(aq) +NO2-1(aq)[ ]initial0.713MXXXX00Δ[ ]- xXXXX+ x+ x[ ]equilibrium(0.713 – x) MXXXXx Mx M
Assuming that “x” is much less than 0.713, the Ka expression simplifies to:
Ka = (x)(x) / (0.713) = 4.0x10-4
x = 0.01689 = [H3O+]
pH = -log[H3O+] = -log(0.01689) = 1.77
At the equivalence point, all of the HNO2(aq) that was originally in the reaction has reacted with OH-1(aq) to form nitrite ions, NO2-1(aq). The titration started with:
(0.03000L HNO2(aq)) (0.713M HNO2(aq)) = 0.02139mols HNO2
So at the equivalence point we have a solution that contains 0.02139mols of NO2-1(aq) in (30.00mL + 28.43mL = 58.43mL) of solution. The concentration of NO2-1(aq) at the equivalence point is:
(0.02139mols of NO2-1(aq)) / (0.05843L) = 0.3659M NO2-1(aq)
This can now be plugged in to a Kb-type equilibrium to solve...
-
NO2-1(aq) +H2O(l) ⇄OH-1(aq) +HNO2(aq)[ ]initial0.3659MXXXX00Δ[ ]- xXXXX+ x+ x[ ]equilibrium(0.3659 – x) MXXXXx Mx M
Assuming that “x” is much less than 0.3659, the Kb expression simplifies to:
Kb = (x)(x) / (0.3659) = 2.5x10-11
x = 3.02x10-6 = [OH-1]
pOH = -log[OH-1] = -log(3.02x10-6) = 5.519
pH = 14 – 5.519 = 8.48
15.00mL of sulfurous acid of unknown concentration is titrated to the second equivalence point with 23.18mL of 0.332M NaOH(aq). What is the concentration of the sulfurous acid stock solution?
H2SO3(aq) + 2 NaOH(aq) ⇄ H2O(l) + Na2SO3(aq)
(0.02318L NaOH(aq)) (0.332M NaOH(aq)) = 7.696x10-3mols NaOH
(7.696x10-3mols NaOH) (1mol H2SO3/ 2mol NaOH) = 3.848x10-3mols H2SO3
(3.848x10-3mols H2SO3) / (0.01500L H2SO3(aq)) = 0.257M H2SO3(aq)
NaOH(aq) should be slightly more concentrated than H2SO3(aq), so this answer is reasonable
I'll be in tomorrow morning, let me know if you have any questions.
2012-07-11
Crunching through K_a problems...
4. What is the expected pH of a 2.49M solution of acetic acid {Ka= 1.8x10-5}? What is the expected pH when 100.0mL of this solution is combined with 100.0mL of water? Assume volumes are additive.
This is a Ka-type equilibrium problem, organize the information using a table.
- HC2H3O2(aq) +H2O(l) ⇄H3O+(aq) +C2H3O2-1(aq)[ ]initial2.49 MXXXX0 M0 MΔ[ ]- xXXXX+ x+ x[ ]equilibrium(2.49 – x) MXXXXx Mx M
Assume “x” is much smaller than 2.49, plug in to the equilibrium constant expression...
x = 6.69x10-3 = [H3O+1]
Assumption is good.
pH = -log[H3O+1] = -log(6.69x10-3) = 2.174
For the second part, the set-up is the same, the only difference is that the initial concentration of acetic acid has been diluted. Calculating the dilution...
C1V1= C2V2
(2.49M)(100.0mL) = C2(200.0mL)
C2= 1.245M
Plug in and solve the same way:
x = 4.73x10-3 = [H3O+1]
Assumption is still good.
pH = -log[H3O+1] = -log(4.73x10-3) = 2.325
5. A 1.83M solution of a weak, monoprotic acid {HA(aq)} has a pH of 3.48. What is the Ka of this acid?
We can approach this as a Ka-type equilibrium problem as well, organize the information using a table.
- HA(aq) +H2O(l) ⇄H3O+(aq) +A-1(aq)[ ]initial1.83 MXXXX0 M0 MΔ[ ]- xXXXX+ x+ x[ ]equilibrium(1.83 – x) MXXXXx Mx M
In this case, we are given a pH, which gives us a way to calculate [H3O+], which gives us “x”...
[H3O+] = 10-pH = 10-3.48 = 3.3113x10-4 (Assumption is good.)
Ka= (3.3113x10-4)(3.3113x10-4) / (1.83) = 5.99x10-8
6. You have combined 100.0mL of 2.84M hydrofluoric acid {Ka = 6.8x10-4} and 100.0mL of 2.19M fluoride ions. What is the expected pH of the resulting solution? Assume volumes are additive.
Again, we can approach this as a Ka-type equilibrium problem (Noticing a pattern here?), organize the information using a table.
- HF(aq) +H2O(l) ⇄H3O+(aq) +F-1(aq)[ ]initial1.42 MXXXX0 M1.095 MΔ[ ]- xXXXX+ x+ x[ ]equilibrium(1.42 – x) MXXXXx M(1.095 + x) M
A couple little adjustments in this case... the original concentrations given in the problem have to be diluted to get the “initial” concentrations in the table. The other key difference here is that we're starting out with a mixture of reactants and products. That might clutter up the math a little bit, but it doesn't really change the way we approach the problem. To simplify things, let's assume that “x” is small compared to both 1.42 and 1.095. Then the Ka expression is:
6.8x10-4= (x)(1.095) / (1.42)
x = 8.818x10-4
Assumption is good.
pH = -log[H3O+1] = -log(8.818x10-4) = 3.055
2012-07-09
Problem 3 (2012-07-09)
14.78g PCl5(g) and 10.15g O2(g) are combined in a 1.500L vessel and reach equilibrium with POCl3(g) and ClO(g). If K = 8.53x10-9, find all equilibrium concentrations.
-
2 PCl5(g) +3 O2(g) ⇄2 POCl3(g) +4 ClO(g)[ ]initial(14.78g / 208.239g/mol)/ 1.500L = 0.047317M(10.15g / 31.998g/mol)/ 1.500L = 0.21147M0 M0 MΔ [ ]– 2x M– 3x+ 2x+ 4x M[ ]equilibrium(0.047317 – 2x) M(0.21147 – 3x) M2x M4x M
Plugging in to the equilibrium constant expression...
Solving this directly would be rough, so let's try a simplifying approximation. Since the equilibrium is quite reactant-favored, we can assume that 2x is small compared to 0.047317 and 3x is small compared to 0.21147. We need to check this later, but that will simplify the equilibrium constant expression to:
x6 = 1.76376x10-16
x = 0.002368
BEFORE WE GO ANY FARTHER, CHECK THE ASSUMPTION WE MADE!! 2x = 0.0047, this is (0.0047/0.047317)*100 = 9.9%. This is a little too high for this assumption to work well. Oops. Don't worry, I don't expect anyone to solve a 6th order polynomial on an exam, these numbers are a little off because I made this problem up during class. For numbers that work, try using K = 8.53x10-12. , then x = 7.489x10-4 and the assumption is OK. If you'd like to know how to solve the original problem, you could use the method of successive approximations, this was how I had to treat these problems when I was an undergrad taking Gen Chem.
Exam tomorrow, if you have questions let me know, I should be online until at least 7 or 8pm tonight.
2012-07-07
Exam 1 and Key posted
Exam 1a and the key are posted at http://www.drbodwin.com/teaching/genchem.php, also linked in the exam archive.
Enjoy the lovely weekend.
Enjoy the lovely weekend.
2012-07-06
Second in-class problem from 2012-07-05
1.92M NF3(g) + 1.63M O2(g) → NOF3(g) Keq=1.74x109, find all [ ]eq
-
2 NF3(g) +O2(g) ⇄2 NOF3(g)[ ]initial1.92 M1.63 M0 MΔ [ ]- 2x M- x M+ 2x M[ ]equilibrium(1.92 – 2x) M(1.63 – 2x) M2x M
Plugging in to the equilibrium constant expression, this would get ugly pretty quickly. Let's try to simplify it with an approximation. Since Keqis quite product-favored, we can probably assume that the limiting reagent is essentially used up to form (almost) the theoretical yield of product. From the balanced chemical equation, we need 2 NF3(g) for each O2(g), but we didn't start with twice as much NF3(g), so that must be the limiting reactant. For every mole of NF3(g) that reacts, 1 mol of NOF3(g) is formed, so the equilibrium concentration of NOF3(g) will be (just a tiny bit less than) 1.92M. To make (just a tiny bit less than) 1.92M NOF3(g), we need halfthat amount of O2(g), so to reach equilibrium we will consume (very close to) 0.96M O2(g), leaving (1.63M – 0.96M = 0.67M) of O2(g) at equilibrium.
{NOTE: If you prefer, you could explicitly calculate this out using our standard 4-step process for limiting reactants/theoretical yields by assuming a volume. I'd assume 1L to make the calculations easier.}
We can now start with a fresh table to organize our information...
-
2 NF3(g) +O2(g) ⇄2 NOF3(g)[ ]equilibrium“a tiny bit”0.67 M1.92 M
Now we can plug into the equilibrium constant expression:
Solving, “a tiny bit” = 5.62x10-5M. Make sure you always CHECK YOUR ASSUMPTIONS! In this case, yes, 5.62x10-5 is indeed very small compared to 1.92, so the assumption is valid. One of the nice things about equilibrium problems is that you can always plug your results back in to the equilibrium constant expression and see if you get an answer that's close to what's given in the problem. In this case...
(1.92)2 / {5.62x10-5)2(0.67) = 1.742x109
So...
[NF3]eq= 5.62x10-5 M
[O2]eq= 0.67M
[NOF3]eq= 1.92M
2012-07-05
First in-class problem from 2012-07-05
2.63M NOCl2(g) → NO(g) + Cl2(g) K=0.118, find all [ ]eq
-
NOCl2(g) ⇄NO(g) +Cl2(g)[ ]initial2.63 M0 M0 MΔ [ ]- x M+ x M+ x M[ ]equilibrium(2.63 – x) Mx Mx M
Plugging in to the equilibrium constant expression...
Because the value of K is not exceptionally large or exceptionally small, this one will probably require working through the quadratic. Breaking it down in steps...
(x)(x) = 0.118(2.63-x)
x2 = 0.31034 – 0.118x
x2 + 0.118x + (-0.31034) = 0
Plugging in to the quadratic formula:
NOTE: The other root is negative, it doesn't work in this problem.
[NOCl2]eq= 2.63 – 0.50 = 2.13M
[NO]eq = x = 0.501M
[Cl2]eq= x = 0.501M
2012-07-02
Problem Set Answers (2012-07-02)
Chem 210 - Summer 2012 - Problem Set {2012-07-02}
1. A 395.4g block of glass (heat capacity = 0.84J/g.K) warms from -42.37℃ to 20.53℃. How much energy/heat has been transferred? Is this process endothermic or exothermic? If the energy/heat gained or lost by the glass block is transferred from/to a 528.7g block of copper (heat capacity = 0.385J/g.K) initially at 15.39℃, what is the final temperature of the copper block?
(0.84J/g•K)(395.4g)(62.90K) = 20891J → 20.9kJ
If the glass block is warming, then energy/heat is being absorbed by the block, this is an endothermic process.(0.385J/g•K)(528.7g)(ΔT) = 20891J
ΔT = 102.6°C
Since the glass block is absorbing heat (endothermic), the copper block must be releasing heat and getting colder.15.39°C – 102.6°C = -87.2°C
2. You have dissolved 12.482g of aluminum nitrate in enough water to make 150.0mL of solution. What is the molarity of aluminum ions in the resulting solution? What is the molarity of nitrate ions in the resulting solution?
First, find the molarity of the whole aluminum nitrate
(12.482g) / (212.994g/mol) = 0.0586026mols Al(NO3)3
(0.0586026mols Al(NO3)3) / (0.1500L) = 0.390684M Al(NO3)3 (aq)
For every mol of Al(NO3)3 that dissolves, there is 1 mol of Al+3(aq). Converting… (0.390684mols Al(NO3)3 / L) (1mol Al+3(aq) / mols Al(NO3)3) = 0.3907M Al+3(aq)
For every mol of Al(NO3)3 that dissolves, there are 3 mol of NO3-1(aq). Converting… (0.390684mols Al(NO3)3 / L) (3mol NO3-1(aq) / mols Al(NO3)3) = 1.1721M NO3-1(aq)
3. What is the freezing point of a solution made from 7.113g of potassium sulfate dissolved in 75.00mL of water?
Calculate the molality of potassium sulfate:
(7.113g) / (174.258g/mol) = 0.040819mols K2SO4
(0.040819mols K2SO4) / (0.07500kg) = 0.54425m K2SO4(aq)
Plugging in to the freezing point depression equation:ΔTfp = kfpd • m • i
ΔTfp = (1.86°C/m)(0.54425m)(3) = 3.04°C
The freezing point of the solution is depressed by 3.04°C. Since the freezing point of pure water is 0°C, the freezing point of the solution should be -3.04°C. 4. Ammonia gas reacts with chlorine gas to form nitrogen trichloride gas and hydrogen gas. At some point, 53.65mg of nitrogen trichloride is formed in 28.63 seconds in a 12.00L reaction vessel. What is the rate of the reaction? What are the rates of consumption and production for each reactant and product in the mixture?
The only thing we have any information about is NCl3, so let’s start by calculating the rate of formation of NCl3 …
RateNCl3 = { (0.05365g NCl3 / 120.366g/mol) / (12.00L) } / 28.63sec = 1.297x10-6 M/sec
Now we need the balanced chemical equation:2 NH3(g) + 3 Cl2(g) → 2 NCl3(g) + 3 H2(g)
Raterxn should be half the rate of production of NCl3 , so (1.297x10-6 M/sec) / 2 = 6.487x10-7 M/sec
RateNH3 should be the same as the rate of production of NCl3 or 2xRaterxn, 1.297x10-6 M/sec
RateCl2 should be three times Raterxn, 3(6.487x10-7 M/sec) = 1.946x10-6 M/sec
RateH2 should be three times Raterxn, 3(6.487x10-7 M/sec) = 1.946x10-6 M/sec
5. You are studying the reaction of sulfur dioxide gas with hydrogen chloride gas to form thionyl chloride {SOCl2(g)} and water gas at 6.48℃. You've completed the following reactions:
| Rxn # | [sulfur dioxide]0 | [hydrogen chloride]0 | Initial Rate (M/min) |
| 1 | 0.429 | 0.317 | 3.02x10-5 |
| 2 | 0.858 | 0.317 | 1.21x10-4 |
| 3 | 0.429 | 0.951 | 9.06x10-5 |
Comparing Rxn #1 and #2, doubling [SO2]0 quadruples the initial rate → 2nd order with respect to [SO2]0
Comparing Rxn #1 and #3, tripling [HCl]0 triples the initial rate → 1st order with respect to [HCl]0
The rate law expression is:
Rate0 = k [SO2]02 [HCl]01
Plugging in conditions for Rxn #1 (or any of them…) to get the value of “k”:3.02x10-5 M/min = k (0.429M)2 (0.317M)
k = 5.176x10-4 M-2min-1
For the new temperature, we need a new value of “k”, plugging in we get…4.67x10-3 M/min = k (0.429M)2 (0.951M)
k = 2.668x10-2 M-2min-1
Plugging in to the comparative form of the Arrhenius equation:ln (k1/k2) = (Ea/R) {(1/T2) – (1/T1)}
ln (5.176x10-4 M-2min-1 / 2.668x10-2 M-2min-1) = (Ea / 8.314J/mol•K) {(1/301.96K) – (1/279.63K)}
Ea = 123.9kJ/mol
Any questions? Let me know.
2012-07-01
Preparing for exams (Summer 2012)
A few people have asked about the best way to prepare for exams this summer. Preparing for exams in summer is really no different than preparing for exams during the regular year, so this advice works for any time.
1. Review class notes and problems we did in class.
2. For topics you feel pretty confident about, take a look at one or two related problems from your textbook to make sure you're in good shape.
3. For topics that you're a little more shaky on, look over the relevant section of your textbook and follow along with the worked out problems in the text. Then try a couple of the end of chapter problems.
4. After you've reviewed the material from class and your textbook, take a look at a previous exam posted on my web page (http://www.drbodwin.com/teaching/examarchive.php) and see if you've got the concepts and problems figured out. Using the exam, identify your weak spots and go back to your textbook to brush up.
5. If you have questions, ask. Check the blog (that's where you are right now!) to see if there is already an answer posted.
The only thing that's slightly different about the summer class is the way the topics are distributed on exams. In a typical spring semester, Exam #1 covers states of matter, gas laws, solution, colligative properties, and maybe a couple other assorted topics. Exam #2 covers kinetics and equilibrium. For the summer class, Exam #1 hits states of matter, gas laws, solution, colligative properties, and kinetics. If you're looking at an Exam #2 from a spring semester and a question seems totally unfamiliar, it might be an equilibrium question, we'll get to that after this week's exam.
We haven't talked about mechanisms and catalysts in class yet, we'll get to those tomorrow (Monday), so they will be on the exam.
Enjoy the rest of your weekend and let me know if you have any other questions.
1. Review class notes and problems we did in class.
2. For topics you feel pretty confident about, take a look at one or two related problems from your textbook to make sure you're in good shape.
3. For topics that you're a little more shaky on, look over the relevant section of your textbook and follow along with the worked out problems in the text. Then try a couple of the end of chapter problems.
4. After you've reviewed the material from class and your textbook, take a look at a previous exam posted on my web page (http://www.drbodwin.com/teaching/examarchive.php) and see if you've got the concepts and problems figured out. Using the exam, identify your weak spots and go back to your textbook to brush up.
5. If you have questions, ask. Check the blog (that's where you are right now!) to see if there is already an answer posted.
The only thing that's slightly different about the summer class is the way the topics are distributed on exams. In a typical spring semester, Exam #1 covers states of matter, gas laws, solution, colligative properties, and maybe a couple other assorted topics. Exam #2 covers kinetics and equilibrium. For the summer class, Exam #1 hits states of matter, gas laws, solution, colligative properties, and kinetics. If you're looking at an Exam #2 from a spring semester and a question seems totally unfamiliar, it might be an equilibrium question, we'll get to that after this week's exam.
We haven't talked about mechanisms and catalysts in class yet, we'll get to those tomorrow (Monday), so they will be on the exam.
Enjoy the rest of your weekend and let me know if you have any other questions.
Subscribe to:
Posts (Atom)