What's the best way to prepare for a cumulative Final Exam? That can be a little ominous, and the "best" way to study can vary considerably from person to person. At this point, you've (hopefully) found something that works for you, whether that means a quiet corner in the newly remodeled library or a lounge with a little more "white noise". Go with the method that works for you!
With that as a foundation, what should you study? It's probably not a good idea to just sit down with the textbook and start reading on page 1. Use the tools you have available to focus and prioritize your studying time and energy!
1. Start with the exams you've already taken!
Look back at your exams. There are things that you knew very well when you took Exam 1 that have gotten a little foggy over the past few months. The good thing is that you knew that material fairly recently, so it'll probably just take a little review to freshen up those concepts and problems.
What about things you didn't do well on earlier exams? Chemistry (and many other fields...) is a cumulative subject. We tend to look at things from a bunch of different directions, and we often approach a concept or problem 2 or 3 different ways over the course of the semester and year. In the past few months, we might have looked at something differently in a way that suddenly makes perfect sense to you.
Use your exams to jot down and prioritize things to study. It won't be a perfect list, but it will give you a good starting point.
2. Review notes from class
Once you've identified the things you need to review, look back at your notes from class.(Sometimes this serves as a reminder to take better notes in the future!) For some topics, a brief reminder from your notes will be all that you need to bring these concepts and problems up from the cold, dark storage room of your memory. For others, it's a good way to once again identify and prioritize your study topics.
3. Use the book
For topics that are still fuzzy, look up key words in the index to help you find a good place to start. Not sure you remember integrated rate laws? Look it up in the index and hit those pages!
It's also not a bad idea to skim over sections that you're pretty sure you understand. Once you're fairly comfortable with a topic, it becomes easier to pick up some of the more subtle points.
End-of-chapter problems can also be helpful because they present information a little bit differently than I do. I'm not talking "better" or "worse" here, the book just uses different wording to ask the same questions I ask. At the end of my class, I don't just want you to be good at answering questions that I write, I want you to understand chemistry, even if someone else is asking the questions.
If you get through all of those steps and still have time, energy, and a thirst for more knowledge, use your favorite tool to search the World Wide Interwebs Net or stop in and chat with me. This isn't a perfect list of study tips, but it's a good place to start. Identify topics, prioritize your studying time and energy, look for connections and common themes in the material, and good luck. You can do it!
Info and advice to help General Chemistry students (and anyone interested in chemistry)
2014-05-02
2014-04-01
More Email Questions...
A couple more email questions:
-----Question-----
On exam b spring 2011 #5 why should this be changed to Ka instead of Kb? Also on exam b spring 2010 #2 the 3rd line its says that HCl works as an effective buffer. I thought an effective buffer had to be a weak conjugate acid/base?
-----Answer-----
Here's question #5
This is Ka because ammonium ion is a conjugate acid. You could also do this as a Kb equilibrium, but you'd be starting with products and shifting to the left to form reactants. Either way should give the same answer.
Here's #2:
Correct, an effective buffer is an approximately equimolar combination of a weak conjugate acid and its weak conjugate base. In the 3rd line here, HCl(aq) is protonating the carbonate in solution 1.5 times so the resulting mixture is 0.64mols HCO3-1(aq) and 0.64mols H2CO3(aq). This is an equimolar mixture of a weak acid and its conjugate base, so it should be a good buffer. {NOTE: because carbonic acid decomposes and the resulting CO2 can escape from solution, this might not be the best buffer in the real world, but it works fine as a sample problem.} This is similar to how you are making the carbonate buffer that you will measure in lab this week.
-----Question-----
On exam b spring 2011 #5 why should this be changed to Ka instead of Kb? Also on exam b spring 2010 #2 the 3rd line its says that HCl works as an effective buffer. I thought an effective buffer had to be a weak conjugate acid/base?
-----Answer-----
Here's question #5
This is Ka because ammonium ion is a conjugate acid. You could also do this as a Kb equilibrium, but you'd be starting with products and shifting to the left to form reactants. Either way should give the same answer.
Here's #2:
Correct, an effective buffer is an approximately equimolar combination of a weak conjugate acid and its weak conjugate base. In the 3rd line here, HCl(aq) is protonating the carbonate in solution 1.5 times so the resulting mixture is 0.64mols HCO3-1(aq) and 0.64mols H2CO3(aq). This is an equimolar mixture of a weak acid and its conjugate base, so it should be a good buffer. {NOTE: because carbonic acid decomposes and the resulting CO2 can escape from solution, this might not be the best buffer in the real world, but it works fine as a sample problem.} This is similar to how you are making the carbonate buffer that you will measure in lab this week.
2014-03-30
Pre-Exam 3 email questions...
A few questions have come in by email, here they are:
----Question-----
----Answer-----
----Question-----
I was going through some of the old exams and the problems that use the Henderson - Hasselbalch. In those examples when it asked for the concentration of the conjugate base over the concentration of conjugate acid, but in the key only the moles of both are put in those places. Why?
----Answer-----
Keep an eye on the weather... Unless MSUM officially closes campus tomorrow, we will have class and the exam as planned. I'll be there at 7:30.
----Question-----
I have a question regarding problem 9 on exam 3a from spring 2013. I understand that the calculated x value doesn't fit under the assumptions. I see where the first two values come from. I was wondering where the -1.238 x 10^-3 came from?
{(x)(x)} / (0.516 – x) = 2.40 x 10^-3
0.516 is the initial concentration and 2.40 x 10^-3 is Ka.
x^2 + (2.40 x 10^-3)x + (-1.238 x 10^-3 ) = 0
In order to use the quadratic formula, we have to solve the equation to the form:
ax2 + bx + c = 0
The (-1.238x10-3) term comes from (2.40x10-3)(0.516).
----Question-----
I was going through some of the old exams and the problems that use the Henderson - Hasselbalch. In those examples when it asked for the concentration of the conjugate base over the concentration of conjugate acid, but in the key only the moles of both are put in those places. Why?
----Answer-----
This is a little mathematical shortcut. Since both the conjugate acid and conjugate base are in the same total volume of solution, the volumes mathematically cancel so I left them out. For example, if we had a buffer made from 0.65mols of HA and 0.55mols of A-1 in 800.0mL of buffer solution, that last part of the Henderson-Hasselbalch would look like:
You can always keep the volume in there and calculate the actual concentrations of each component, you should get exactly the same answer either way.
----Question-----
I dont understand how you can derive pH values from pka's given in the question. I also dont understand how pH can be calculated at each eq point in the titration curve as in number 11 on spring 2013 where it asks what indicator to use.. it says use the 2 pkas...how does this help?
----Answer-----
I dont understand how you can derive pH values from pka's given in the question. I also dont understand how pH can be calculated at each eq point in the titration curve as in number 11 on spring 2013 where it asks what indicator to use.. it says use the 2 pkas...how does this help?
----Answer-----
There are a couple ways that pKa (or pKb) can lead to a pH. One possibility is in a question like "What is the expected pH of a 0.618M solution of ammonium nitrate solution?" In this question, you can set up a Ka-type equilibrium for ammonium ions and use the Ka of ammonium to calculate [H3O+] and pH. This is similar to the problem I posted yesterday (http://chemistryingeneral.blogspot.com/2014/03/neutral-salts.html). This method can be used to calculate the initial pH for a titration. It would also work to approximate the pH of an equivalence point. Let's think about that...
For the titration of phosphite ions with hydrochloric acid, we can calculate the initial pH by setting up a Kb-type equililbrium and using the Kb of phosphite ion to calculate [OH-1] and pOH and pH. At the first equivalence point in this titration, we have a solution that we can think of as HPO3-2(aq) because we have added just enough acid to complete the following equation exactly once:
PO3-3(aq) + H+(aq) <=> HPO3-2(aq)
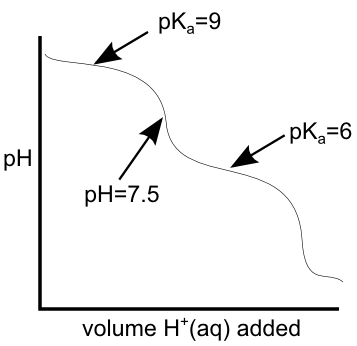
Between equivalence points, we have buffering regions of the titration curve... at the mid-point of this buffering region, the pH is equal to the pKa of the weak acid of the mixture. If we know the pKa (and therefore the pH) on either side of the equivalence point we're interested in, we can get a pretty reliable estimate of the pH of that equivalence point. On the titration curve below, if the pKas that bound the equivalence point are 6 and 9, the pH of the equivalence point should be right between them at pH = 7.5.Keep an eye on the weather... Unless MSUM officially closes campus tomorrow, we will have class and the exam as planned. I'll be there at 7:30.
2014-03-29
Neutral salts
The conjugate of a strong acid or strong base is neutral. That's just something we tend to accept, memorize, and move on. But why? Those two words are a big part of the reason I'm a chemist.
Let's take a look at a strong acid and see if we can make sense of this. Of the typical strong acids, nitric is usually the weakest, and nitric is also the only one that might have a Ka listed in standard tables. The stronger strong acids have really useful Ka values listed in the tables like "large" or "strong"... I don't have a "large" button on my calculator, so let's just use nitric acid and we'll hopefully see why the other strong acids would follow the same trend if we had a value for their Ka.
The Ka for nitric acid is usually listed at around 25. That means the Kb for nitrate ions is:
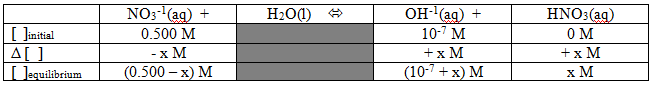
That's a REALLY weak Kb, but we can go ahead and calculate the pH of a solution just like in any other situation. How about the problem: What is the expected pH of a 0.500M solution of sodium nitrate? {By the way, we could make similar arguments to show that the sodium ions don't affect the pH, but we'll save those for another day...} As with all good equilibrium problems, it's probably not a bad idea to start with a table:
Now we can set up the Kb expression and plug in the numbers we have:
We should be able to simplify that with some assumptions... Let's assume that "x" is much smaller than 0.500 and much larger than 10-7. That gets us the simplified expression:
Solving this expression, we get x = 1.41x10-8, which gives us a pOH = -log(1.41x10-8) = 7.85, and pH = 6.15. Hmm, that's not neutral, that's acidic. The whole point of this was to prove that nitrate was a neutral ion. This is a disaster.
BUT WAIT!
We made some assumptions. We didn't check our assumption after we solved for "x". This is why I always tell you to check assumptions... We assumed that "x" would be much smaller than 0.500, which it is, but we also assumed that "x" was much larger than 10-7, which it absolutely is not! So the assumptions we made were an oversimplification of the problem and that's where we entered the danger zone. Looking back at our Kb expression, we can only simplify it to:
That's still going to require the quadratic formula to solve. I'll let you work out the details, but the result should be that x = 7.96x10-9. That means:
Let's take a look at a strong acid and see if we can make sense of this. Of the typical strong acids, nitric is usually the weakest, and nitric is also the only one that might have a Ka listed in standard tables. The stronger strong acids have really useful Ka values listed in the tables like "large" or "strong"... I don't have a "large" button on my calculator, so let's just use nitric acid and we'll hopefully see why the other strong acids would follow the same trend if we had a value for their Ka.
The Ka for nitric acid is usually listed at around 25. That means the Kb for nitrate ions is:
That's a REALLY weak Kb, but we can go ahead and calculate the pH of a solution just like in any other situation. How about the problem: What is the expected pH of a 0.500M solution of sodium nitrate? {By the way, we could make similar arguments to show that the sodium ions don't affect the pH, but we'll save those for another day...} As with all good equilibrium problems, it's probably not a bad idea to start with a table:
Now we can set up the Kb expression and plug in the numbers we have:
We should be able to simplify that with some assumptions... Let's assume that "x" is much smaller than 0.500 and much larger than 10-7. That gets us the simplified expression:
Solving this expression, we get x = 1.41x10-8, which gives us a pOH = -log(1.41x10-8) = 7.85, and pH = 6.15. Hmm, that's not neutral, that's acidic. The whole point of this was to prove that nitrate was a neutral ion. This is a disaster.
BUT WAIT!
We made some assumptions. We didn't check our assumption after we solved for "x". This is why I always tell you to check assumptions... We assumed that "x" would be much smaller than 0.500, which it is, but we also assumed that "x" was much larger than 10-7, which it absolutely is not! So the assumptions we made were an oversimplification of the problem and that's where we entered the danger zone. Looking back at our Kb expression, we can only simplify it to:
That's still going to require the quadratic formula to solve. I'll let you work out the details, but the result should be that x = 7.96x10-9. That means:
[OH-1]eq = 10-7 + (7.96x10-9) = 1.08x10-7 M
pOH = -log(1.08x10-7) = 6.9667
pH = 14 - 6.9667 = 7.0333
That's not exactly 7.0000000000 neutral, but it's pretty darn close, especially if we're thinking about this in terms of selecting a visual acid-base indicator for a titration.
2014-01-19
Quiz 2 Hint
Quiz 2 has a heat capacity problem, and the heat capacity value given in the problem is in units of "joules per (mole Kelvin)". Remember, for heat capacity problems, we're looking at changes in temperature. Whether it's Celsius or Kelvin, the ΔT is the same because 1 degree C and 1 K are exactly the same size. You can use either, but make sure you're doing everything in the right order. A temperature that changes from 3.45°C to 12.71°C is changing by:
ΔT = 12.71°C - 3.45°C = 9.26°C
If you prefer Kelvin, convert BOTH individual temperatures to K, THEN subtract:
ΔT = 285.86K - 276.60K = 9.26K
Other questions, let me know...
Quiz 1 questions...
I've gotten a few questions about Quiz 1. Here are a few hints...
Question 1: How many moles of nitrogen atoms are in a given mass of iron(III) nitrate?
Start with a balanced chemical formula. How many moles of N are in each mole of iron(III) nitrate? Use the mole ratio to get moles of N from the moles of iron(III) nitrate.
Question 2: How many moles of chlorine atoms are in a given volume of a given concentration vanadium(IV) chlorate solution?
This is almost the same as question 1, but you need to use concentration and volume to determine the moles of vanadium(IV) chlorate instead of mass and molar mass.
Two other helpful hints:
1. Don't wait until the day before a quiz is due to work on it. This is especially true because...
2. When you have a question, show me your work. If you have the problem all set up, it's much easier for me to look at what you've done and help you work through the problem correctly. It's always easier to help you when you can give me an idea of where you're getting confused.
Question 1: How many moles of nitrogen atoms are in a given mass of iron(III) nitrate?
Start with a balanced chemical formula. How many moles of N are in each mole of iron(III) nitrate? Use the mole ratio to get moles of N from the moles of iron(III) nitrate.
Question 2: How many moles of chlorine atoms are in a given volume of a given concentration vanadium(IV) chlorate solution?
This is almost the same as question 1, but you need to use concentration and volume to determine the moles of vanadium(IV) chlorate instead of mass and molar mass.
Two other helpful hints:
1. Don't wait until the day before a quiz is due to work on it. This is especially true because...
2. When you have a question, show me your work. If you have the problem all set up, it's much easier for me to look at what you've done and help you work through the problem correctly. It's always easier to help you when you can give me an idea of where you're getting confused.
2014-01-12
Spring Semester 2014 is here!
We're 18 hours away from the first Gen Chem II class of 2014! Watch this blog for general info about the topics we'll be exploring and answers to specific questions I get by email. I'll also be tweeting links and daily summaries using #GenChem2014. And I might try a few other things this semester…
A couple tips that are always useful:
1. If there's a way to calculate moles of a substance, that might be a good start.
2. Balance your chemical formulas. Then balance your chemical reactions. Balance is the key to chemistry. And skiing. If you don't balance your formulas and reactions, you're gonna have a bad time.
3. Be curious. That what science is all about.
4. Ask questions. Unless you're the only person in the room, it's pretty likely that someone else in the room has the same question you do.
5. Be bold. It's OK to answer a question incorrectly in class, we're learning chemistry here not juggling chainsaws. The other side of this is to be supportive of your classmates, we're all here to learn (including me).
Enjoy the beautiful weather today and I'll see you tomorrow.
A couple tips that are always useful:
1. If there's a way to calculate moles of a substance, that might be a good start.
2. Balance your chemical formulas. Then balance your chemical reactions. Balance is the key to chemistry. And skiing. If you don't balance your formulas and reactions, you're gonna have a bad time.
3. Be curious. That what science is all about.
4. Ask questions. Unless you're the only person in the room, it's pretty likely that someone else in the room has the same question you do.
5. Be bold. It's OK to answer a question incorrectly in class, we're learning chemistry here not juggling chainsaws. The other side of this is to be supportive of your classmates, we're all here to learn (including me).
Enjoy the beautiful weather today and I'll see you tomorrow.
Subscribe to:
Posts (Atom)