Let's take a look at a strong acid and see if we can make sense of this. Of the typical strong acids, nitric is usually the weakest, and nitric is also the only one that might have a Ka listed in standard tables. The stronger strong acids have really useful Ka values listed in the tables like "large" or "strong"... I don't have a "large" button on my calculator, so let's just use nitric acid and we'll hopefully see why the other strong acids would follow the same trend if we had a value for their Ka.
The Ka for nitric acid is usually listed at around 25. That means the Kb for nitrate ions is:
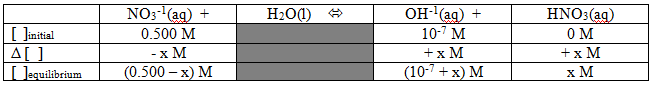
That's a REALLY weak Kb, but we can go ahead and calculate the pH of a solution just like in any other situation. How about the problem: What is the expected pH of a 0.500M solution of sodium nitrate? {By the way, we could make similar arguments to show that the sodium ions don't affect the pH, but we'll save those for another day...} As with all good equilibrium problems, it's probably not a bad idea to start with a table:
Now we can set up the Kb expression and plug in the numbers we have:
We should be able to simplify that with some assumptions... Let's assume that "x" is much smaller than 0.500 and much larger than 10-7. That gets us the simplified expression:
Solving this expression, we get x = 1.41x10-8, which gives us a pOH = -log(1.41x10-8) = 7.85, and pH = 6.15. Hmm, that's not neutral, that's acidic. The whole point of this was to prove that nitrate was a neutral ion. This is a disaster.
BUT WAIT!
We made some assumptions. We didn't check our assumption after we solved for "x". This is why I always tell you to check assumptions... We assumed that "x" would be much smaller than 0.500, which it is, but we also assumed that "x" was much larger than 10-7, which it absolutely is not! So the assumptions we made were an oversimplification of the problem and that's where we entered the danger zone. Looking back at our Kb expression, we can only simplify it to:
That's still going to require the quadratic formula to solve. I'll let you work out the details, but the result should be that x = 7.96x10-9. That means:
[OH-1]eq = 10-7 + (7.96x10-9) = 1.08x10-7 M
pOH = -log(1.08x10-7) = 6.9667
pH = 14 - 6.9667 = 7.0333
That's not exactly 7.0000000000 neutral, but it's pretty darn close, especially if we're thinking about this in terms of selecting a visual acid-base indicator for a titration.




No comments:
Post a Comment