----Question-----
I have a question regarding problem 9 on exam 3a from spring 2013. I understand that the calculated x value doesn't fit under the assumptions. I see where the first two values come from. I was wondering where the -1.238 x 10^-3 came from?
{(x)(x)} / (0.516 – x) = 2.40 x 10^-3
0.516 is the initial concentration and 2.40 x 10^-3 is Ka.
x^2 + (2.40 x 10^-3)x + (-1.238 x 10^-3 ) = 0
In order to use the quadratic formula, we have to solve the equation to the form:
ax2 + bx + c = 0
The (-1.238x10-3) term comes from (2.40x10-3)(0.516).
----Question-----
I was going through some of the old exams and the problems that use the Henderson - Hasselbalch. In those examples when it asked for the concentration of the conjugate base over the concentration of conjugate acid, but in the key only the moles of both are put in those places. Why?
----Answer-----
This is a little mathematical shortcut. Since both the conjugate acid and conjugate base are in the same total volume of solution, the volumes mathematically cancel so I left them out. For example, if we had a buffer made from 0.65mols of HA and 0.55mols of A-1 in 800.0mL of buffer solution, that last part of the Henderson-Hasselbalch would look like:
You can always keep the volume in there and calculate the actual concentrations of each component, you should get exactly the same answer either way.
----Question-----
I dont understand how you can derive pH values from pka's given in the question. I also dont understand how pH can be calculated at each eq point in the titration curve as in number 11 on spring 2013 where it asks what indicator to use.. it says use the 2 pkas...how does this help?
----Answer-----
I dont understand how you can derive pH values from pka's given in the question. I also dont understand how pH can be calculated at each eq point in the titration curve as in number 11 on spring 2013 where it asks what indicator to use.. it says use the 2 pkas...how does this help?
----Answer-----
There are a couple ways that pKa (or pKb) can lead to a pH. One possibility is in a question like "What is the expected pH of a 0.618M solution of ammonium nitrate solution?" In this question, you can set up a Ka-type equilibrium for ammonium ions and use the Ka of ammonium to calculate [H3O+] and pH. This is similar to the problem I posted yesterday (http://chemistryingeneral.blogspot.com/2014/03/neutral-salts.html). This method can be used to calculate the initial pH for a titration. It would also work to approximate the pH of an equivalence point. Let's think about that...
For the titration of phosphite ions with hydrochloric acid, we can calculate the initial pH by setting up a Kb-type equililbrium and using the Kb of phosphite ion to calculate [OH-1] and pOH and pH. At the first equivalence point in this titration, we have a solution that we can think of as HPO3-2(aq) because we have added just enough acid to complete the following equation exactly once:
PO3-3(aq) + H+(aq) <=> HPO3-2(aq)
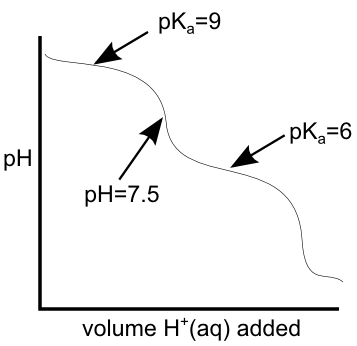
Between equivalence points, we have buffering regions of the titration curve... at the mid-point of this buffering region, the pH is equal to the pKa of the weak acid of the mixture. If we know the pKa (and therefore the pH) on either side of the equivalence point we're interested in, we can get a pretty reliable estimate of the pH of that equivalence point. On the titration curve below, if the pKas that bound the equivalence point are 6 and 9, the pH of the equivalence point should be right between them at pH = 7.5.Keep an eye on the weather... Unless MSUM officially closes campus tomorrow, we will have class and the exam as planned. I'll be there at 7:30.

No comments:
Post a Comment